Preceyes received CE mark for eye surgery
A nice example of idea-to-product development is presented by Preceyes, which is finally now cleared in Europe. We have covered the system eariler here, here and here.
"Preceyes B.V. has received CE marking approval for its PRECEYES Surgical System R1.1 to assist trained surgeons during vitreoretinal surgical tasks in patients requiring vitreoretinal intervention under local or general anesthesia. The use of the robotic assistant is anticipated to not only improve treatment quality to be provided to patients, it also offers the possibility of developing completely new treatments.
The PRECEYES Surgical System R1.1 is the world’s first robot that has been clinically validated to assist surgeons in retinal surgery. With the CE mark, the robotic system is now available for wider commercial deployment in Europe and can be readily integrated at major hospitals and institutes for clinical and research activities. The system has a proprietary design and is developed in collaboration with clinicians to user requirements.
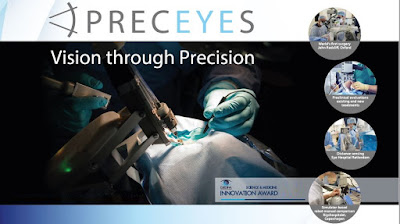
The robotic manipulator of the PRECEYES Surgical System holds and positions a surgical instrument inside the eye. The system allows surgeons to operate on retinal structures down to as little as 20 microns in size for prolonged periods of time, compared to the standard physiological limit of 100 microns for manual surgery. Furthermore, the system is able to automate motion profiles, including limiting instrument movement. Surgeons cannot offer this type of high-precision surgery to their patients with manual surgery.
“Retinal surgeries demand a high degree of precision and steadiness,” said Prof. Marc de Smet M.D., Chief Medical Officer of Preceyes. “The precision of our robotic assistance will enable better treatment quality as well as the possibility to develop new treatments. It represents the most advanced option in eye care available to date. While we are focusing on vitreoretinal applications at the moment, we believe the system can be developed to assist surgeons in performing most surgical procedures in the eye.”
Gerrit Naus, Chief Executive Officer of Preceyes said: “This CE mark is the first approval by a regulatory agency for the use of a robotic system in retinal surgery and confirms our position as the world leader in robot-assisted retinal eye surgery. The CE marking approval is an important milestone for Preceyes. We will leverage the CE marking approval for further industrialization of our technology and commercial deployment in Europe, developing partnerships for accessing markets worldwide.”
Further information
Gerrit Naus, Chief Executive Officer of Preceyes B.V., +31 40 247 4789, contact@preceyes.nl. For more information on the PRECEYES Surgical System, visit www.preceyes.nl.
About Preceyes B.V.
Preceyes is a medical robotics company focused on ocular surgery. The company develops, builds and commercializes innovative robotic solutions to assist eye surgeons in performing the most demanding surgical tasks. The company’s first target is vitreoretinal surgery. The robot supports the surgeon in improving existing surgery and enables the development of new, high-precision treatments. www.preceyes.nl"
The PRECEYES Surgical System R1.1 is the world’s first robot that has been clinically validated to assist surgeons in retinal surgery. With the CE mark, the robotic system is now available for wider commercial deployment in Europe and can be readily integrated at major hospitals and institutes for clinical and research activities. The system has a proprietary design and is developed in collaboration with clinicians to user requirements.
The robotic manipulator of the PRECEYES Surgical System holds and positions a surgical instrument inside the eye. The system allows surgeons to operate on retinal structures down to as little as 20 microns in size for prolonged periods of time, compared to the standard physiological limit of 100 microns for manual surgery. Furthermore, the system is able to automate motion profiles, including limiting instrument movement. Surgeons cannot offer this type of high-precision surgery to their patients with manual surgery.
“Retinal surgeries demand a high degree of precision and steadiness,” said Prof. Marc de Smet M.D., Chief Medical Officer of Preceyes. “The precision of our robotic assistance will enable better treatment quality as well as the possibility to develop new treatments. It represents the most advanced option in eye care available to date. While we are focusing on vitreoretinal applications at the moment, we believe the system can be developed to assist surgeons in performing most surgical procedures in the eye.”
Gerrit Naus, Chief Executive Officer of Preceyes said: “This CE mark is the first approval by a regulatory agency for the use of a robotic system in retinal surgery and confirms our position as the world leader in robot-assisted retinal eye surgery. The CE marking approval is an important milestone for Preceyes. We will leverage the CE marking approval for further industrialization of our technology and commercial deployment in Europe, developing partnerships for accessing markets worldwide.”
Further information
Gerrit Naus, Chief Executive Officer of Preceyes B.V., +31 40 247 4789, contact@preceyes.nl. For more information on the PRECEYES Surgical System, visit www.preceyes.nl.
About Preceyes B.V.
Preceyes is a medical robotics company focused on ocular surgery. The company develops, builds and commercializes innovative robotic solutions to assist eye surgeons in performing the most demanding surgical tasks. The company’s first target is vitreoretinal surgery. The robot supports the surgeon in improving existing surgery and enables the development of new, high-precision treatments. www.preceyes.nl"
Source: Preceyes


Comments
CE Certification
iso 27001 malaysia
CE Certification Cost
CE Certification
iso 14001 training
ce certification
CE Certification in Sri Lanka
iatf 16949 certification
iso 13485 indonesia
ce certification in sri lanka