CE mark for avatera, the first German system for RAMIS
Yesterday it was announced that the first entirely German RAMIS system received CE mark - the avatera.
"Jena, Germany, November 14, 2019 – avateramedical
GmbH, an innovative German medical technology company, today announced
the successful completion of the CE conformity assessment procedure for
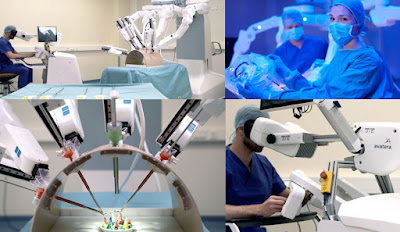
its avatera® system1 for robot-assisted, minimally
invasive surgery. All four components of the system – the surgical
robot and control unit, the instruments, the endoscopes and the sterile
components – can now carry the CE mark. This means that avatera is now
approved in the European Economic Area for minimally invasive
laparoscopy (“key hole surgery”), to this point primarily used in
gynecology and urology.
The avatera system was developed based on current standards in
robot-assisted surgery and optimized in close cooperation with future
users – including surgeons and surgical teams – in terms of cost,
quality, comfort and reliability. avatera's unique single-use concept
for instruments and sterile components guarantees functional, reliable
instruments for every surgical procedure, reduces the risk of
contamination and associated patient infections, and avoids the cleaning
and sterilization necessary with reusable instruments. Compared to
classical laparoscopy, the success of robot-assisted surgery is based on
the high level of comfort, precision and dexterity of the instruments.
With its open design and low noise level, the avatera system also
enables smooth communication in the operating room.
“The successful completion of the CE conformity assessment is an important milestone in our young history,” commented Dr. Hubertus von Gruenberg, co-founder and CEO of the avateramedical
group. “We are very proud to offer our 'German Robot', which is
developed and produced in the two Thuringian sites of the Company – Jena
and Ilmenau – as a competitive solution in this strongly growing
market. In the next step, we will introduce the avatera system to
clinical practice.” At the same time, avateramedical's
management announced its strategic plan to build one of the most modern
and largest production facilities for medical robotics in Germany in
2020 to support increased demand anticipated over the next five years.
“We are confident in our plan to launch avatera in 2020, aspiring to
significantly improve the uptake and access to robot-assisted surgery in
Europe,” concluded Dr. von Gruenberg.
The global market for surgical robots currently stands at USD 4.5
billion with experts estimating growth to USD 13 billion by 2025.
Current statistics underpin this growth potential with about 8.5
robotic systems (also called telemanipulator systems) available per 1
million inhabitants in the U.S. compared to only 1.0 surgical robots per
1 million inhabitants in Europe. With an ageing population, increasing
demands on hospital efficiency and the growing need of surgeons and
patients for better technologies, robotic surgery could soon become the
gold standard in European hospitals.
About avatera
avatera® is the first German system for robot-assisted, minimally invasive surgery (MIS). Tailored precisely to the needs of future users, "The German Robot" enables precise key hole surgery (so-called laparoscopy) with the highest level of safety for patients and maximum comfort for surgeons and surgical teams. The use of German servers and the application of German and European data protection standards ensure maximum security for the protection of all sensitive data of clinics and patients. A certified quality management system and the CE mark document the complete conformity of avatera with all legal and normative requirements and confirm the high standards avateramedical applies to the safety and efficiency of the system.
avatera® is the first German system for robot-assisted, minimally invasive surgery (MIS). Tailored precisely to the needs of future users, "The German Robot" enables precise key hole surgery (so-called laparoscopy) with the highest level of safety for patients and maximum comfort for surgeons and surgical teams. The use of German servers and the application of German and European data protection standards ensure maximum security for the protection of all sensitive data of clinics and patients. A certified quality management system and the CE mark document the complete conformity of avatera with all legal and normative requirements and confirm the high standards avateramedical applies to the safety and efficiency of the system.
avateramedical GmbH is an innovative German medical
technology company in the field of robot-assisted surgery at the
Thuringian high-tech sites Jena and Ilmenau. The privately financed
company was founded in 2011 by Prof. Dr. med. Jens-Uwe Stolzenburg and
Dr. Hubertus von Gruenberg and financed by Lars Windhorst to combine the
expertise of leading European surgeons, German top managers and
excellent German engineers and software developers. avateramedical,
with its current workforce of around 130 employees, aims to combine
state-of-the-art medical technology with economic efficiency, quality,
comfort and reliability. avateramedical GmbH is a subsidiary of avateramedical N.V.
For more information, please see https://www.avatera.eu"
Source: avatera


Comments
AS we know Covid-19 virus now spread also by air so please stay home and take care all.
Nice and Thanks for an information based post.These tips are really helpful. Thanks a lot.Keep it up.Keep blogging.!! I am very happy to visit your site to know really very important topics by your such a nice blog post.
text messages service
text message business
business text messages
text for business
gateways sms
send text messages
online text message sender
online send sms
text sms
sms for marketing
sms marketing services
bulk sms
bulk text message
sms service provider
CE Mark
CE Certification in UAE