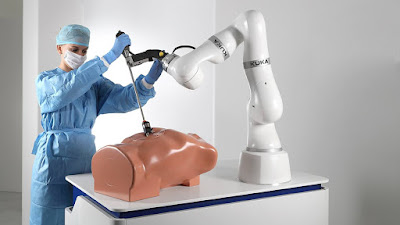
KUKA LBR Med lightweight robot
The LBR Med - now in serial production - is the first stand-alone robot manipulator that meets the regulatory requirements of IEC 60601-1 (Medical Device Directive in Europe).
"KUKA has made extensive adaptations to enable
operation of the innovative lightweight robot under the strict
requirements of medical treatment and interventions. It has
biocompatible and corrosion-resistant surfaces. Internally routed
connections offer a further point of compliance with the hygienic
standards in doctors’ offices, clinics and operating rooms. KUKA also
guarantees inspection of the LBR Med through the IECEE CB scheme
according to standard IEC 60601-1. With the CB Certificate and CB Report
from KUKA, complex testing is reduced and certification procedures are
made significantly easier. KUKA already takes care of this in the
factory.
As a robot component for a vast range of tasks, the robot can be
integrated into medical products by the medical product manufacturer.
Equipped with the right tools and program, the LBR Med can be used to
assist with endoscopy or biopsies. It can be used to saw bones or for
the fixation of pedicle screws. The sensitive seven-axis robot is based
on revolutionary lightweight robot technology from KUKA. It has been
completely redefining human-robot collaboration for years."
Source: KUKA Roboter


Comments
Biocompatibility Testing Services
Biocompatibility For Medical Devices
Neonatal Warmer
Neonatal Incubator
Delivery Table
Baby Warmer
labour table
neonatal cpap