Revo-i approved in Korea
Following an 7-year-log development period, the Revo-i system of Meere Company (previously called Eterne) finally got cleared in Korea. It has been used in clinical trails for two years.
"The Ministry of Food and Drug Safety (MFDS) has approved Revo-i, the first surgical robot system developed in Korea, officials said Thursday.
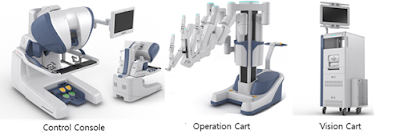
Revo-i is a system in which a physician inserts a robot arm into the
body of the patient after making a minimum incision, and surgeons can
view three-dimensional images inside. Revo-i is used for general
endoscopic surgery, including cholecystectomy and prostatectomy. In
particular, this product uses four robotic arms to grasp the surgical
site, cut and seal.
The surgical robots market is increasing because they can accurately
recognize surgical sites and minimize the incision through 3D
stereoscopic images. The global robot import market is expected to grow
by 12.1 percent a year to 9.64 trillion won ($8.33 billion) by 2021.
Korea’s imports of surgical robots totaled 19.6 billion won last year, up 34 percent from 15.6 billion won in 2015.
The successful localization of surgical robots will likely reduce
financial burdens of patients who need endoscopic surgery and speed up
their recovery by reducing blood loss."
Their early clinical results on a porcine model have just been published.
Source: Korea Biomed


Comments